Of the seven 14-3-3 isoforms present in mammalian cells most are ubiquitously expressed while some of them exhibit tissue specific expression. E.g. 14-3-3ε and 14-3-3γ are expressed in most cell types and are the most evolutionarily conserved of the 14-3-3 isoforms, which is consistent with their role in regulating cdc25C function. In contrast, 14-3-3σ is only expressed in epithelial cells. 14-3-3σ expression is decreased in multiple epithelial tumour types, especially in tumours of the breast and loss of 14-3-3σ leads to immortalization in keratinocytes and in the growth of breast tumours. All these results are consistent with the hypothesis that 14-3-3σ might function as a tumour suppressor in tumours derived from multiple epithelial tissues.
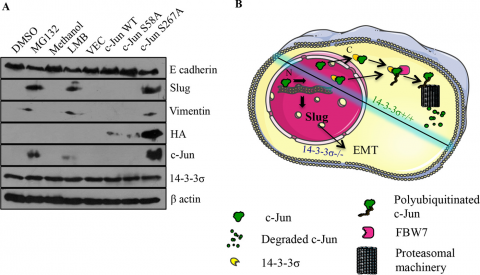
Our results have also demonstrated that loss of 14-3-3σ in HCT116 cells leads to the induction of an Epithelial Mesenchymal Transition (EMT) due to the stabilisation of c-Jun thus inducing the expression of the EMT transcription factor, slug. One of the features of the EMT observed upon 14-3-3σ loss is a decrease in PKP3 expression and this is accompanied by an increase in invasion and migration, phenotypes also observed upon PKP3 loss. However, in contrast to cells with a knockdown of PKP3, cells with a loss of 14-3-3σ formed tumours with lower efficiency in immunocompromised mice and did not show metastatic progression (57). This led to the question of why two different genetic events with similar outcomes in the same cells led to vastly different phenotypes and whether we can use these cell systems to identify mechanisms that are essential for tumour progression and metastasis. Further, as the loss of 14-3-3σ also leads to increased radio and chemo sensitivity, we are currently investigating the mechanisms underlying the increased sensitivity to therapy observed upon the loss of 14-3-3σ.